EXTENDING A PIPELINE INTO THE FUTURE OF SKIN HEALTH
At Dermavant, we’re leading the way for change by targeting the unmet needs of people with chronic skin conditions. We leverage groundbreaking innovations to develop products that HCPs will want to prescribe and patients will want to use.
About AhR
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcriptional regulatory complex that controls the expression of a wide range of genes involved in maintaining a stable environment. In particular, AhR has roles in maintaining homeostasis in barrier tissues such as the skin, lung, and gut.1
THE AhR SIGNALING PATHWAY
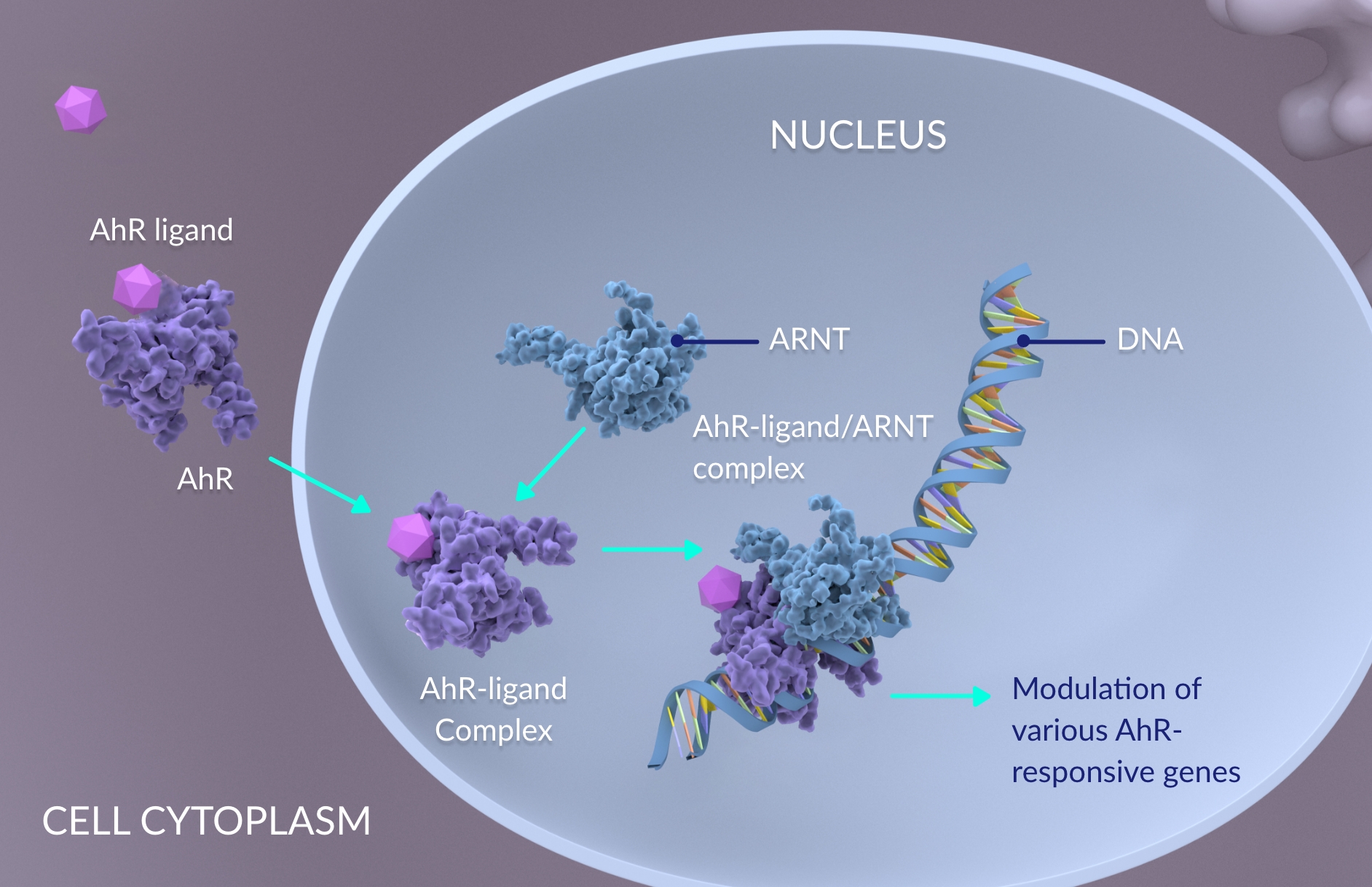
From extracellular ligand to gene expression
- Signaling begins when a ligand binds to AhR in the cell cytoplasm, forming an AhR-ligand complex1
- The AhR-ligand complex translocates to the nucleus and heterodimerizes with the AhR nuclear translocator (ARNT) to form a high affinity DNA-binding transcriptional regulatory complex2,3
- The activated AhR-ligand/ARNT complex then interacts with DNA to promote modulation of various AhR-responsive genes1–3
EXAMPLES OF BARRIER TISSUES
SKIN4
- Regulates activation of T cells and antigen-presenting cells
- Normalizes the expression of pivotal barrier proteins and terminal differentiation of keratinocytes
- Protects against oxidative stress
LUNGS4
- Regulates inflammatory and immune responses
GUT4
- Important for the development of intestinal epithelium
- Maintenance of intestinal epithelial integrity and regulation of innate gut immune cells.
Available Product
Approved Indication
Stage of Development
Preclinical
Phase 1
Phase 2
Phase 3
FDA Review
Commercial
VTAMA® (tapinarof) cream, 1% (DMVT-505)
A topical aryl hydrocarbon receptor (AhR) agonist
Plaque Psoriasis in Adults
Progress value is 100%
100%VTAMA® (tapinarof) cream, 1% (FDA approved)
Indication: Plaque Psoriasis in Adults
A post-marketing phase 4 study of VTAMA cream in adults with plaque psoriasis in the in intertriginous areas (i.e., skin folds).
Read Press ReleaseVisit ClinicalTrials.gov
A post-marketing phase 4 study of VTAMA cream in adults with plaque psoriasis in the head and neck regions.
Read Press ReleaseVisit ClinicalTrials.gov
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
Product Candidate
Potential Indication
Stage of Development
Preclinical
Phase 1
Phase 2
Phase 3
FDA Review
Commercial
Tapinarof (DMVT-505)
A topical aryl hydrocarbon receptor (AhR) agonist
Atopic Dermatitis
Progress value is 82%
82%Tapinarof cream, 1%
Indication: Atopic Dermatitis
A maximal usage study of tapinarof cream in children ages 2 to 17 years with atopic dermatitis.
Read Press ReleaseVisit ClinicalTrials.gov
Topline results from the Phase 3 ADORING 1 and ADORING 2 program.
Read Press ReleaseVisit ClinicalTrials.gov
DMVT-506
Next-generation aryl hydrocarbon receptor (AhR) agonist under development for multiple routes of administration
Immunological and Inflammatory Diseases
Progress value is 10%
10%DMVT-506
Indication: Immunological and Inflammatory Diseases
An early-stage drug candidate currently in development as a potential treatment for immunological and inflammatory diseases.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
Product Candidate
Indication
Stage of Development
Preclinical
Phase 1
Phase 2
Phase 3
FDA Review
Commercial
VTAMA® (tapinarof) cream, 1% (DMVT-505)
A topical aryl hydrocarbon receptor (AhR) agonist
Plaque Psoriasis in Adults
Progress value is 100%
100%VTAMA® (tapinarof) cream, 1% (FDA approved)
Indication: Plaque Psoriasis in Adults
A post-marketing phase 4 study of VTAMA cream in adults with plaque psoriasis in the in intertriginous areas (i.e., skin folds).
Read Press ReleaseVisit ClinicalTrials.gov
A post-marketing phase 4 study of VTAMA cream in adults with plaque psoriasis in the head and neck regions.
Read Press ReleaseVisit ClinicalTrials.gov
Tapinarof (DMVT-505)
A topical aryl hydrocarbon receptor (AhR) agonist
Atopic Dermatitis
Progress value is 64%
64%Tapinarof cream, 1%
Indication: Atopic Dermatitis
A maximal usage study of tapinarof cream in children ages 2 to 17 years with atopic dermatitis.
Read Press ReleaseVisit ClinicalTrials.gov
Topline results from the Phase 3 ADORING 1 and ADORING 2 program.
Read Press ReleaseVisit ClinicalTrials.gov
DMVT-506
Next-generation aryl hydrocarbon receptor (AhR) agonist under development for multiple routes of administration
Immunological and Inflammatory Diseases
Progress value is 10%
10%DMVT-506
Indication: Immunological and Inflammatory Diseases
An early-stage drug candidate currently in development as a potential treatment for immunological and inflammatory diseases.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
References:
1. Esser C, Rannug A. Pharmacol Rev. 2015;259-275.
2. Furue M, et al. J Dermatol Sci. 2015;80:83-88.
3. Bissonnette R, et al. J Am Acad Dermatol. 2021;84:1059-1067.
4. Esser C, et al. Pharmacol Rev. 2015;67:259-279.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
You are now leaving Dermavant.com
You are now leaving www.dermavant.com. Links to sites outside of Dermavant are provided as a resource to the viewer. Dermavant accepts no responsibility for the content of linked sites.
Important Safety Information
Indication: VTAMA® (tapinarof) cream, 1% is an aryl hydrocarbon receptor agonist indicated for the topical treatment of plaque psoriasis in adults. Adverse Events: The most common adverse reactions (incidence ≥ 1%) in subjects treated with VTAMA cream were folliculitis (red raised bumps around the hair pores), nasopharyngitis (pain or swelling in the nose and throat), contact dermatitis (skin rash or irritation, including itching and redness, peeling, burning, or stinging), headache, pruritus (itching), and influenza (flu).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.



